ATLANTIS is a clinical trial testing whether a tablet called amitriptyline helps people with irritable bowel syndrome (IBS).
Our video gave an introduction to the ATLANTIS trial for potential participants. You can also download the video transcript.
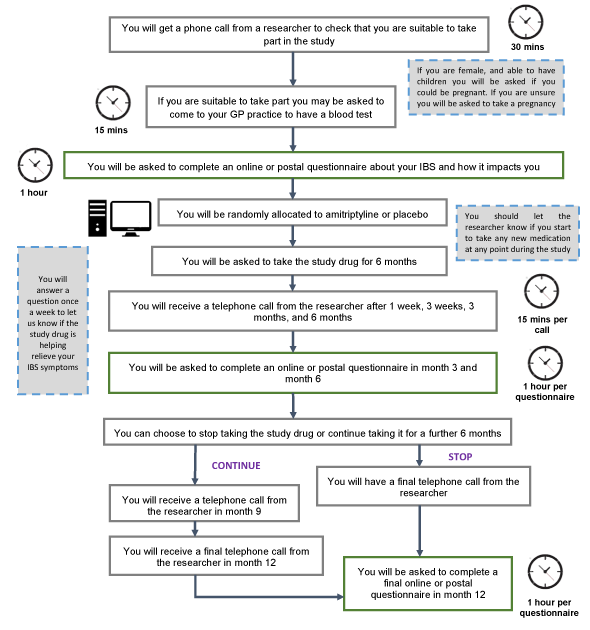
What happened if you took part in the ATLANTIS trial?
1. Screening Call
To make sure you are suitable to take part, you will have an initial telephone call with a researcher to find out more about you and your IBS.
2. Eligibility Tests
If you are eligible to take part, you may be asked to come into your GP practice and have some blood samples taken.
If you are a woman, able to have children, and are unable to confirm you are not pregnant, you will be provided with a pregnancy test to use at home before going into the study. This is to ensure that it is safe for you to receive amitriptyline in the study.
3. Baseline Questionnaire
You will be asked to complete an online or postal questionnaire about your IBS and how it impacts you. You will then be randomly allocated to amitriptyline or placebo.
4. Drug Treatment
You will be asked to take the study drug for 6 months. After 6 months, you can choose to stop taking the study drug, or take it for a further 6 months.
5. Phone Calls
Once you start taking the study drug, you will receive a telephone call from the researcher after:
- 1 week
- 3 weeks
- 3 months
- 6 months
This is to give you advice about your study medication and check if there are any problems, as well as to answer any questions you may have.
If you decide to take the study drug for 12 months, the researcher will also call you in month 9.
6. Questionnaires
You will complete an online or postal questionnaire at:
- 3 months
- 6 months
- 12 months
You will also answer a question once a week to let us know if the study drug is helping you. This can be answered online or can be recorded in a paper diary.
7. End of Treatment
You will have a telephone call with the researcher when you finish taking the study drug to check if there are any problems and to answer any questions you may have.
8. Trial Summary