ARTEMIS

Augmenting RadioTherapy in REctal Cancer to
Minimise Invasive Surgery
Hello and thank you for taking the time to visit the ARTEMIS website. Here you will find useful information about the ARTEMIS trial.
If you are taking part in the trial, we would like to say a huge THANK YOU for your help in developing ways in which we can improve cancer treatments.
We will post a new ARTEMIS newsletter to the website every 6 months to keep you updated with the latest information on the trial. Alternatively, if you prefer a hardcopy your nurse will be happy to provide you with one at your next hospital appointment. We have also provided you a link to the Key Facts sheet and Patient Information Sheet to read again if you so wish.
Newsletters
Background
The ARTEMIS trial is for patients who have been diagnosed as having a cancer of the lower bowel, known as the rectum. There are no definite metastases. At present, surgery is the standard treatment for rectal cancer. Some patients require radiotherapy or chemoradiotherapy to reduce the size of the cancer prior to surgery. In some cases, this treatment may be enough to treat the cancer completely and surgery may be avoided.
We want to find out if giving an additional drug treatment, AN0025, alongside radiotherapy and chemotherapy, could improve the chance of the cancer completely responding to treatment and therefore increasing the number of patients who can avoid surgery. We hope this will also be associated with an improved quality of life for patients.
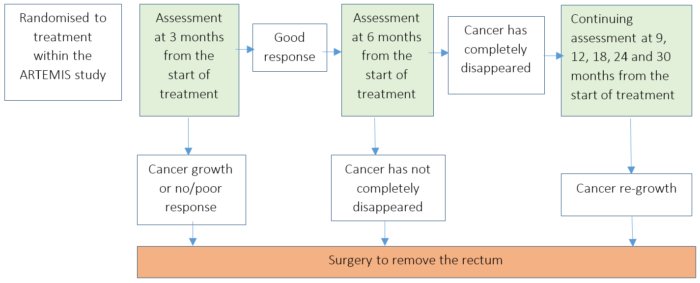
Trial Schema
After randomisation to receive either the radiotherapy and chemotherapy or the addition of AN0025 alongside these treatments, you will follow the treatment schedule below:
Long Course

Short Course

Trial treatment
- The study will aim to recruit 140 volunteers with rectal cancer who are interested in the chance of getting a new trial treatment with the hope of getting rid of the cancer without the need for surgery or a colostomy (stoma).
- In this study everybody will receive chemoradiotherapy (radiotherapy and oral capecitabine) followed by 3 months of chemotherapy (CAPOX/FOLFOX). Half of the patients will also be randomised to receive an additional oral immunotherapy, called AN0025. ‘Randomised’ means that a computer will select at random whether or not the participant receives the immunotherapy agent and not the treating clinician.

After treatment
- We will continue to monitor the progress of participants once their treatment has ended. This will likely take the form of a telephone appointment a few weeks after treatment ends followed by regular appointments at 4, 6, 9, 12, 18, 24 and 30 months following the start of treatment.
Key Factsheets
Long Course Chemoradiotherapy (LCCRT)
FOLFOX v1.0
CAPOX v1.0
Short Course Radiotherapy (SCRT)
FOLFOX v1.0
CAPOX v1.0
Contact
General enquiries
[email protected]
Professor Simon Gollins
Consultant Clinical Oncologist
Co-Chief Investigator
[email protected]
Professor Mark Saunders
Consultant Clinical Oncologist
Co-Chief Investigator
[email protected]
Dr Samantha Noutch
Senior Trial Coordinator
Clinical Trials Research Unit
[email protected]



